How Lithium ion battery works
The perception in the public domain is that lead acid batteries are old technology. Lithium ion battery has a different perception, it is modern, cleaner, it has 3 or 4 times the energy density and longer cycle life. With all of this, what possible advantages could the 150-year old lead acid technology bring to the table? Well actually, all is not as it seems, look behind the headlines at the data used in the marketing claims, then apply a bit of common sense, basic research and some rudimentary science. You will find that the real story is rather different.
The first misconception concerns the volumetric and specific energy densities. The headline values of 4 to 5 times relate only to the specific energy density and to a limited number of lithium ion battery chemistries, some of which are still not in commercial use. Fig. 2 compares several cathodes for lithium ion battery cells these range from around 100Wh/kg for the safest Li-FePO4 chemistry to over 200Wh/kg for the nickel-cobalt-aluminum oxide variant. Lead acid battery diagram is given below:


These values only apply to the single-cell level, not the pack or in-service condition. Fig. 3 shows the energy densities of different battery chemistries at cell and system level. The energy densities of lithium ion battery cells are practically halved when fully installed with all the connections, cooling, safety and battery management equipment.
The cell level advantage of 3 to 5 times the specific energy density is reduced to 2 to 3 times. Dependent upon the lithium cathode chemistry we could almost be looking at parity between lithium ion batteries and lead acid batteries energy density for a fully installed battery system in some applications.
The other factor, that of cycle life, is also a source of confusion. How many cycles can a lithium ion battery perform before the capacity drops below 80% of its nameplate rating? Two, three thousand? Table 1 gives a summary of the different Li-ion cathode materials for performance and cycle life.
Advantages of Lead acid battery chemistry
Batteries are strange devices. Nobody wants them, but everybody needs them. They are only bought when required. How many people plan a trip to the local mall to window shop for batteries? They are a grudge purchase and only bought when absolutely necessary. A good salesman can sell you two pair of shoes, two cars and maybe two houses if you have the money, but he cannot sell you two SLI automobile batteries. When you do buy a battery whether a solar battery for a solar panel, an electric bike or a UPS and inverter battery backup system or a traction battery for forklifts don’t you wish you knew more about it?
How do lead acid batteries work, what is the differences between types and models, and how about the different chemistries? They can be expensive. In a commercial or domestic application what is the payback, what is the life and the cost of replacement of a lead acid battery? The size you need, the space available, the energy efficiency of lead acid battery and recharge time? And then, there is the hidden costs of safety, disposal and the carbon footprint. This article compares lead acid batteries with lithium ion battery and addresses many of the misconceptions associated with both of these chemistries.
Which lithium ion battery is best
| Cathode material | Short name | Nominal voltage | Specific energy Wh/kg (cell) | Cycle life | Comments |
|---|---|---|---|---|---|
|
Lithium Cobalt Oxide (LiCoO2) |
LCO | 3.6 | 150-200 | 500-1000 | Portable devices - thermal runaway on overcharge |
| Lithium Manganese Oxide (LiMn2O4) | LMO | 3.7 | 100-150 | 300-700 | Power tools, medical devices - safer than LCO |
| Lithium Nickel Manganese Cobalt Oxide (LiNiMnCO2) | NMC | 3.6/3.7 | 150-220 | 1000-2000 | E-bikes, EV, industrial - high cycle life |
| Lithium Iron Phosphate (LiFePO4) | LFP | 3.2 | 90-120 | 1000-2000 | EV, SLI, Leisure - safest of all lithium ion battery chemistries |
| Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) | NCA | 3.6 | 200-260 | 500 | Industrial, EV powertrain (Tesla) TR at 150C, CL 500 |
| Lithium Titanate (Li4Ti5O12) | LTO | 2.4 | 50-80 | UPS, Solar, EV powertrain (Honda, Mitsubishi). CL 3000-7000 - very safe |
As can be seen, all fall within the 800 to 2000 cycle range. In comparison, a well-designed lead acid battery can easily achieve more than 1600 cycles to 80% DOD. So how does this all add up when considering the cost of ownership? This brings us to the next point which is the lead-acid battery price. How much does a lithium-ion battery cost compared to a lead-acid battery? Lithium-ion battery manufacturing plant cost? Naturally, lithium ion battery is more expensive but how much more. Again, this depends upon the level being considered. The press releases will tell us that Li-ion prices are falling and now are in the range of 2-3 times that of lead acid.
Really? The mean prices on a recent UK internet search to obtain prices on commercially available leisure batteries of 12V and 100 Ah for both lithium ion battery and lead acid batteries:
lithium ion battery $960 or $800/kwh
Lead acid battery $215 or $180/kwh
Obviously, the life of the lithium ion battery has to be 4 times that of the lead acid battery equivalent to get the same value. As we have seen, this is not the case.


In all cases, lead-acid battery construction was the most cost-effective even when a larger lead-acid battery was fitted to give better charge acceptance and longer cycle life. In this example, the application was a telecoms tower in India. The same principle holds true in most applications and geographies, more so in colder climates. The other misconception is that Li-ion is a cleaner technology and less polluting than lead-acid. The cradle to gate emissions for different battery chemistries is given in Figs. 5 and 6.
This figure shows the boundary of operations for battery manufacture. From the extraction and transport of raw materials right through all the processing steps to the point where the batteries are ready to ship.
Table 2 is a real-life situation comparing the economics of using lithium ion battery and lead acid battery working over different life periods.
| Cost item | Daily running costs USD | Daily running costs USD |
|---|---|---|
| 3 Years | Lead Acid Battery | Lithium ion battery |
| Amortisation | 8.30 | 16.90 |
| Diesel (delivered) | 15.50 | 15.50 |
| Maintenance | 2.46 | 2.46 |
| Electricity | 1.47 | 1.47 |
| Battery Charging | 0.65 | 0.50 |
| Total day/month | 28.38/851 | 36.83/1105 |
| 6 Year | ||
| Amortisation | 5.86 | 8.46 |
| Diesel | 15.50 | 15.50 |
| Maintenance | 2.46 | 2.46 |
| Electricity | 1.47 | 1.47 |
| Battery Charging | 0.54 | 0.50 |
| Total day/month | 25.83/775 | 28.39/852 |
This data from Argonne National Laboratories, show that the total manufacturing process including the extraction and transport of raw materials for lithium ion batteries are more than 4 times the lead acid value. Regarding the extraction of materials, the supply of basic cathode materials such as cobalt and manganese and lithium are not completely certain. The extraction and recovery processes exist but the number of mines and manufacturing sites may limit supply if demands significantly increases. The geo-political map also predicts uncertainty for some sources of these materials.
Are lithium ion battery recyclable
The recyclability and safety of these chemistries are important factors. It is known that almost all of the components in lead acid batteries are 100% recycled whereas there are no commercial processes for recycling lithium ion battery. This situation is understandable when you consider that the more expensive components of Li, Co, Mn etc. are only a small fraction of the total lithium ion battery. For example, Lithium is around 4% of the total cell weight. Add to this the obvious fact that Lithium is highly reactive (the basis of its high energy density), which understandably makes it expensive to extract from the waste.
The additional factor of complexity with many different materials in its construction makes recycling difficult, both technically and economically. The result? There is simply no commercial incentive to recycle these batteries. For this reason, recycling facilities are still at the pilot stage and mostly government-funded.
At present, the vast majority of scrapped lithium ion battery are stockpiled waiting for either a technological breakthrough or legislation to force their recycling. If the latter were to be implemented then there would be a cost, ultimately to the consume. This would further increase the price of the Li-ion cell compared with lead acid battery types.
Can lithium ion battery explode
Finally, we have safety. No lead acid battery applications to our knowledge has ever had a safety recall as we know is the case with Li-ion battery in portable electronic devices and even electric vehicles. Fig. 7 shows what happened to a new hybrid Volvo in the UK just a couple of weeks ago, at the time of writing this article. In this case its lithium ion batteries caught fire when on charge.
Lithium-ion battery fires
Figure 7 Fire caused by a Li-ion battery in a Volvo hybrid electric vehicle: April 2018-UK residence


This video shows a very recent fire caused by a lithium battery. Possibly due to imbalance in cells and improper BMS.
Even when stored or transported lithium ion batteries have been the cause of seriously dangerous fires. Whilst these occasions are rare, they have to be acknowledged, and suitable safety equipment and battery management software have to be installed. The New York fire department for example are still in the process of deciding how to tackle lithium ion batteries fires. This would strongly suggest that existing safety measures for lithium ion batteries worldwide need to be reviewed.
Following is the view from the New York Fire Department:
News article quote: AWS utility drive Nov. 15, 2016 “Fire is not the biggest problem,” said Rogers. Firefighters are trained to deal with fires, but they need to know what they are dealing with. Li-ion batteries can release toxic acids and flammable vapours. Some of those vapours are consumed by the fire, but if they are not, they could ignite or be a problem for firefighters. The biggest problem is what happens “post-op,” that is after the fire is extinguished. Even if a battery is shut down it could reignite for up to 72 hours, Rogers said. -Lt. Paul Rogers Fire Department of New York’s hazardous materials operations division”
Lithium ion battery or Lead acid battery?
Lithium ion battery most certainly has better performance characteristics than lead acid. However, these advantages are severely reduced by the additional hardware associated with the safety and management requirements. The net result is that lead acid batteries have distinct advantages, particularly when considering applications which are not restricted by weight or charge acceptance. The lower initial cost of lead acid battery manufacturing plant cost; the low purchasing price and low amortization cost of lead acid combined with its low environmental impact and inherent safety, provide the following advantages:
- Lower purchase price. The price is around one-quarter of a Li-ion equivalent. The lower operating costs to give a lower total cost of ownership in the majority of applications.
- Recyclability. Almost 100% of all lead acid battery materials are recycled. The scrap value can provide additional revenue of up to 20% of the battery material cost. Lithium batteries have no infrastructure or commercial process for recycling
- Safety. The chemistry of lead acid is inherently safer than that of lithium ion battery
- Sustainability. There are many well-established sources of supply for lead acid, particularly from recycling facilities. Lithium and other cathode materials may be supplied from politically sensitive areas. Both the current global materials extraction and manufacturing capability would not support a rapid increase in the production of Lithium ion battery.
- Carbon footprint. Lead acid battery manufacture has a cradle to gate carbon footprint one-third of that of lithium ion batteries.
A different picture to the one painted by the lithium ion battery companies. Whilst it cannot be argued that lead acid has a disadvantage in energy density, the fact is lead-acid battery is still a highly safe, competitive and the best choice of battery technology in many applications.
What's lithium ion battery
Cathode and Anode materials: Although nickel-metal hydride (Ni-MH) cells were favoured initially in the 1990s, the world’s first commercial lithium ion rechargeable battery product was released in 1991 by Sony Corporation. In addition to high energy content, both by mass and volume, this battery also offered excellent low-temperature characteristics, load characteristics and cycle characteristics. As a result, it quickly captured the market and became an indispensable source of power for audio and video equipment, personal computers, portable telephones, and other portable equipment
Today’s advanced battery technology started with the discovery of the high ionic conductivity of the solid phase NaAl11O17, called sodium β-alumina, by Kummer and co-workers at the Ford Motor Co. laboratory [1. Olof Ramsrtomström, on Nobel prize for Chemistry, Scientific Background on the Nobel Prize in Chemistry 2019; 2. Y.F.Y. Yao and J.T. Kummer, J. Inorg. Nucl. Chem. 29, 2453 (1967)].
This led to the realization that ionic transport in solids can actually be very fast, and that it might lead to a variety of new technologies. Shortly thereafter, researchers at Ford showed that one can use a highly conducting solid electrolyte to produce an entirely new type of battery, using molten sodium at the negative electrode and a molten solution of sodium in sulfur as the positive electrode, with the sodium-conducting solid electrolyte in between [N. Weber and J.T. Kummer, Proc. Annual Power Sources Conf. 21, 37 (1967) ].
As might be expected, consideration was soon given to the possibility of analogous lithium systems, for it was recognized that an otherwise equivalent lithium cell should produce higher voltages than a sodium cell. In addition, lithium has a lower weight than sodium, another advantage.
Elemental lithium could not be used, because of its low melting point. Instead, solid lithium alloys, primarily the Li/Si and Li/Al systems, were investigated [R.A. Huggins, J. Power Sources 81–82, 13 (1999)].
A number of materials were investigated as positive electrode reactants at that time, with most attention given to the use of either FeS or FeS2. Upon reaction with lithium, these materials undergo reconstitution reactions, with the disappearance of the initial phases and the formation of new ones [D.R. Vissers, Z. Tomczuk and R.K. Steunenberg, J. Electrochem. Soc. 121, 665 (1974)].
When was lithium ion battery invented?
Prof. Whittingham explored electrochemical intercalation in such materials and in 1973 proposed such materials as electrodes in batteries. This work resulted in a working, rechargeable battery in 1976. The successful cell was composed of lithium metal as the anode and titanium sulfide (TiS2) as the cathode, with lithium hexafluorophosphate (LiPF6) as the electrolyte in propylene carbonate (PC) as the solvent. These promising studies inspired Whittingham to explore electrochemical intercalation in such materials as electrodes in batteries. A working, rechargeable battery was subsequently demonstrated in 1976
[(a)Whittingham, M. S. Electrointercalation in Transition-Metal Disulphides. J. Chem. Soc., Chem. Commun. 1974, 328–329.] (with Exxon Research and Engineering Company).
(b)Whittingham, M. S. Batterie à Base de Chalcogénures. Belgian patent no. 819672, 1975.
(c)Whittingham, M. S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192 (4244), 1126–1127.
But the success was short-lived. On repeated cycling, metallic lithium formed dendrites at the metal surface on cycling, resulting in short-circuits.
This problem gave impetus to a new search for alternative solutions and an “ion transfer cell” configuration (also called “rocking chair”) cells, where both electrodes can accommodate ions was proposed.
If a positive electrode material initially contains lithium and some or all of the lithium is removed during first charging, the cell develops potential. Therefore, it is possible to have positive electrode materials that react with lithium at potentials above about 3V, if they already contain lithium, and this lithium can be electro-chemically extracted.
Who invented lithium ion battery?
This approach, involving the use of materials in which lithium is already present, was first demonstrated by Prof. Goodenough. The first examples of materials initially containing lithium, and electrochemically deleting lithium from them, were the work on Li1−xCoO2 in 1980.
[K. Mizushima, P.C. Jones, P.J. Wiseman and J.B. Goodenough, Mater. Res. Bull. 15, 783 (1980)] and Li1−xNiO2
[J.B. Goodenough, K. Mizushima and T. Takada, Jpn. J. Appl. Phys. 19 (Suppl. 19-3), 305 (1980)]
In parallel with the anode development, better cathode materials were also sought after in order to acquire a higher cell emf in combination with anodes of higher potential than metallic lithium. A breakthrough came in 1979/1980 when John B. Goodenough and his co-workers at Oxford
University, UK, discovered that LixCoO2, another intercalated metal chalcogenide of type MX2, could serve as a cathode material.
[Goodenough, J. B.; Mizushima, K. Fast Ion Conductors. US patent no. 4,357,215, 1982].
[Mizushima, K.; Jones, P. C.; Wiseman, P. J.; Goodenough, J. B. LixCoO2 (0<x<-1): A New
Cathode Material for Batteries of High Energy Density. Mater. Res. Bull. 1980, 15 (6), 783–789].
The structure of the material was analogous to LixTiS2 with van der Waals gaps between the cobalt dioxide (CoO2) layers in which lithium ions could be bound without much lattice expansion. Goodenough reasoned that when X in MX2 is a small electronegative element, a resulting cation uptake process would be associated with a large negative free-energy change and a high cell voltage (ΔG = -nFE). With an X of oxygen, the situation was deemed especially promising, also given that lithium ions were proposed to be sufficiently mobile in close-packed oxygen arrays.
The reasoning proved to be correct, and the CoO2 material showed a very high potential of ~4 to 5 V relative to Li+/Li. The electrochemical studies were carried out in this case with an electrolyte composed of lithium tetrafluoroborate (LiBF4) in propylene carbonate.
This discovery enabled the use of anode materials with higher potentials than lithium metal, furthering the search for suitable carbonaceous materials. Considering the difficulty of solving the problem of the electrochemical intercalation of graphite, other options were investigated instead.
Where was the lithium ion battery invented?
A breakthrough came in 1985 when a Japanese group led by Akira Yoshino (of Asahi Kasei Corporation) discovered vapour-phase-grown carbon fibbers (VGCF) and later heat-treated petroleum coke. The latter material was known to contain a mixture of crystalline (graphitic) and non-crystalline domains, and the researchers could identify particularly stable, yet high-performing, qualities with specific degrees of crystallinity.
[Akira Yoshino, The Birth of Li-Ion Battery, Angewandte Essays, Angew., Chem. Int. Ed., 2012, 51, 5798-5800]
With these effective anode materials, Yoshino developed an efficient, working lithium-ion battery based on the ion transfer cell configuration. The identified carbonaceous material was thus used as an anode and Goodenough’s LixCoO2 material (typically containing small amounts of tin) was used as a cathode. Separator layers composed of polyethylene or polypropylene were used and the electrolyte was composed of lithium perchlorate (LiClO4) in propylene carbonate (PC).
Yoshino also proved the safety of this battery in 1986 by dropping a weight on the battery. No fires or explosions occurred whereas batteries using lithium metal anode reacted violently.

Figure 8. Yoshino’s first safety tests with his Li-ion battery in 1986.
A) The moment an iron lump collides with the battery
B) Prototype Li-ion battery after collision
C) Metallic Li anode battery after collision
[Credit: Akira Yoshino, The Birth of Li-Ion Battery, Angewandte Essays, Angew., Chem. Int. Ed., 2012, 51, 5798-5800 ]
These discoveries and developments ultimately led to the release of a commercial lithium battery
in 1991. With further development, the Li-ion battery was commercialised by Sony in 1991 and by a joint venture of Asahi Kasei and Toshiba in 1992.
[Nishi, Y., The Development of Lithium Ion Secondary Batteries. Chem. Rec. 2001, 1, 406–413]
The battery was based on a petroleum coke–based anode material, LixCoO2 as the cathode, and a water-free electrolyte composed of lithium hexafluorophosphate (LiPF6) in propylene carbonate (PC). The charging voltage was high (up to 4.1 V), with a recorded specific energy of ~80 Wh/kg and energy density of ~200 Wh/ litre.
Compared to other batteries that were on the market at the time, the lithium battery quickly became very competitive and essentially paved the way for the upcoming mobile revolution.
At around the same time, it was found that graphite could actually be used in combination with a suitable electrolyte composition. [Fong R, Sacken U von, Dahn J. R., Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137 (7), 2009–2013]
By using solvents containing ethylene carbonate, hitherto generally disregarded due to its higher melting point, a solid electrolyte interphase (SEI) was formed at the surface of the graphite electrode during the charge/discharge cycle, thereby protecting the carbon material from exfoliation and further decomposition. [Peled, E. The Electrochemical Behaviour of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems, The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126 (12), 2047–2051.
This discovery was rapidly adopted by the battery community, and a next-generation lithium-ion battery based on graphite as the anode material was developed. With this anode material, batteries with charging voltages of 4.2 V were produced soon after, resulting in the energy density of ~400 Wh/litre.
The development of the lithium-ion battery did not stop with these important discoveries, but many improvements and alternatives have since been reported. For example, new cathode materials have continuously been identified for use in specific battery applications, and two such materials have originated from Goodenough’s group: the spinel material Li1-xMn2O4 and the olivine material LixFePO4 (LFP).
[Padhi, A. K.; Nanjundaswami, K. S.; Goodenough, J. B. Phospho-Olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194.
Thackeray, M. M.; David, W. I. F.; Bruce, P. G.; Goodenough, J. B. Lithium Insertion into Manganese Spinels. Mater. Res. Bull. 1983, 18, 461–472].
The latter material is limited by a somewhat lower potential versus Li+/Li than LixCoO2, but has high stability and can be used at high charging rates. Several other electrode materials and electrolyte systems have also been discovered, leading to ever-improved energy storage materials for the benefit of society.
What kind of battery is used in electric vehicles?
Nowadays, most of the EVs use Li-ion batteries. Earlier, Ni-MH and lead-acid batteries were used, but their usage slowly declined due to the advent of Li-ion batteries, which possess higher specific energy and higher energy density values. The specific energy of lead acid batteries is about 40-50 Wh/kg while the Li-ion battery has about 150 Wh/kg. The energy density value for lead-acid batteries is 80-100 Wh/litre while Li-ion battery has got more than 250 Wh/litre.
Cylindrical cells with nickel-cobalt-aluminium (NCA) cathodes and silicon/graphite composite anodes, such as those used in the latest Tesla battery packs (2019-2020), have reached approximately 270 Wh/kg and 650 Wh/litre. A new technology called Licerion by Sion Power claims 500 Wh/kg specific energy and 1000 Wh/L energy density and >450 cycles in 0.4 Ah development cells.
For small batteries, we speak in terms of Wh. For higher capacity systems, kWh unit is used. The Wh value divided by 103 will give the kWh.
Thus 850 Wh = 850/1000 = 0.850 kWh.
Cells used in today’s EV battery can reach nominal specific energy of 140 -170 Wh/kg. The specific energy of the resulting battery pack is typically 30 to 40 per cent lower, or 80 -120 Wh/kg. The reduction is due to several series and parallel connecting leads, BMS and thermal management system (cooling or heating). In 2019, the pack percentage of non-cell components has come down to about 28 %.
Until now, cells were first put into modules and then put into packs. Both Contemporary Amperex Technology Co. Limited, China (CATL) and Tesla have decided that they want to get rid of the modules and place the cells into packs directly. CATL has already done so and calls it cell-to-pack technology. While information about this is scarce, the company claims that this can increase specific energy by 10–15% and improve volume utilization by 15–20%. In total, it can reportedly reduce the parts needed for the battery packs by 40%. [https ://cleantechnica.com/2020/02/18/how-catl-lithium-iron-phosphate-batteries-could-be-leading-to-100-kwh-tesla-model-3/]
Designation of Lithium Battery
The International Electrotechnical Commission (IEC) and the Indian Standards Institution have established a common designation to describe the chemistry and size of Lithium-ion cells.
[Secondary lithium cells and batteries for portable applications, International Electrotechnical Commission, IEC 61960-1 and IEC 61960-2 and IS 16047: 2012].
The letters designate the chemistry and form factor while the numbers specify the physical dimensions of the cell. The first letter describes the general chemistry, the second letter designates the specific cathode chemistry and the third letter designates the shape.
First letter: I – Lithium-ion chemistry
Second letter: C- cobalt, F- iron, Fp – iron phosphate, N- nickel, M-manganese, Mp- manganese phosphate, T- titanium, V –vanadium and x – others.
Third letter: R- cylindrical, P-prismatic
The first two numbers that follow designate the diameter in mm and the last three to designate the height in tenths of mm. Thus a cell designated ICR19/66 is a Lithium ion cell with a cobalt cathode that has a diameter which is > 18 mm and ≤ 19 mm and a maximum overall height which is > 65 mm and ≤ 66 mm.
For prismatic cells the initial letters have the same meaning but the first two numbers designate width in mm, the next two numbers are the height in mm and the last two numbers are the length in mm. Thus, a cell designation IMP9/35/150 describes a prismatic Lithium ion cell with a manganese cathode cell whose maximum thickness is > 8 mm and ≤ 9 mm and a maximum width which is > 34 mm and ≤ 35 mm and a maximum overall height which is > 149 mm and ≤ 150 mm.
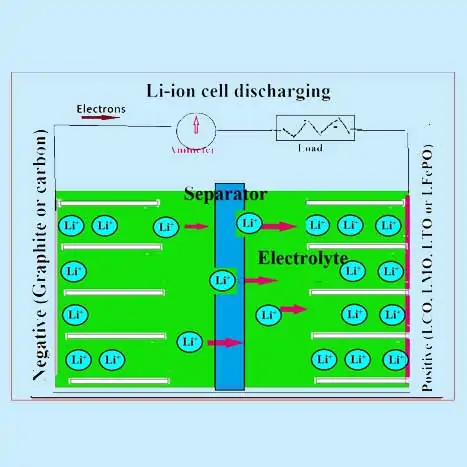
How Does a Lithium-ion Battery Work?
how lithium ion battery is made
Lithium metal with atomic number 3, density of 0.534 g/cc, having very low standard reduction potential (Li+/Li couple -3.05 V vs. SHE) and theoretical specific capacity of 3860 Ah/kg (2061 mAh/cc) is the lightest weight, highest voltage, and greatest energy density of all metals. (Compare with lead of atomic number 82, density 11.29 g/cc, theoretical specific capacity of 257.8 Ah/kg and standard reduction potential of -0.35V vs. SHE).
Lithium ion battery - Active Materials
The active materials of the positive electrode are any one of the mixed oxides like LiCoO2 or LiMnO2 or LiFePO4. The negative electrode is mainly graphite and amorphous carbon compounds. An organic electrolyte (containing a dissociated lithium conducting salt such as LIPF6) is used. A polypropylene (PP) or polyethene (PE) or mixed separator is used. Lithium ions migrate back and forth between the electrodes of lithium-ion batteries during charging and discharging and are intercalated into the active materials as described below:

Figure 9. An exploded view of a Lithium ion cell
Credit: Zhang Z., Ramadass P. (2012) Lithium-Ion Battery Systems and Technology. In: Meyers R.A. (eds) Encyclopaedia of Sustainability Science and Technology. Springer, New York, NY, pp 6124. http s://doi.org/10.1007/978-1-4419-0851-3_663
How lithium ion battery charge
During a discharge process in a Lithium ion cell (LIB) lithium ions from the anode are de-intercalated (or extracted) into the electrolyte and these lithium ions from the electrolyte are intercalated into the cathode material. This movement of the ions from anode to cathode is accompanied by the release of electrons which flows in the external circuit. The reverse process occurs during the charging process where lithium ions move from the cathode and intercalate in the anode through the electrolyte. Commercial LIBs typically use transition metal oxides such as LiCoO2, LiMn2O4, and LiFePO4 as the cathode material, which is coated over an aluminium current collector.
Ten to twenty per cent of conductive carbon and 5%–10% of polymeric binders like polyvinylidene difluoride (PVDF) and polytetrafluoroethylene (PTFE) are also added along with active material to enhance the electronic conductivity and achieve better adhesion of the electrode material, respectively. The anode material is coated over a copper current collector with conducting carbon and PVDF if required.
The two electrodes are separated by a porous separator (polyethylene or polypropylene film of thickness 10–20 µm) soaked in an electrolyte solution (LiPF6 in an organic solvent). Both separator and electrolyte solution should have better ionic conductivity. The cell is usually fabricated in a metal casing in jellyroll fashion with an electrolyte-dipped separator in between the two electrodes. A schematic of a LIB is shown in the figures, where typical charge and discharge processes are shown.
Lithium-ion (Li-ion) rechargeable batteries employ a reversible insertion/extraction of lithium ions (Li+) (guest species) into or from a host matrix (positive and negative electrode active materials) called lithium insertion compounds when the discharge and charge processes occur. Lithium ion batteries have been referred to as rocking chair batteries because the lithium ions “rock” back and forth between the positive and negative electrodes as the cell is charged and discharged.
The positive active material is typically a metal oxide with a layered structure, such as lithium cobalt oxide (LiCoO2), or a material having a tunnelled structure, such as lithium manganese oxide (LiMn2O4), mostly on an aluminium current collector. The negative active material is typically a graphitic carbon, also a layered material, mostly on a copper current collector. In the charge-discharge process, lithium ions are inserted or extracted from interstitial space between atomic layers of the active materials.
Non-aqueous electrolytes or organic electrolytes are used in lithium cells.
Separators for lithium-ion batteries are polyolefin microporous films polyethylene (PE) and polypropylene (PP).

Electrochemical cell reactions in lithium ion battery
In a typical Lithium ion cell, the following generic reactions occur.
Positive electrode reaction:
LiMO2 ⇔ Li1-xMO2 + x Li+ + x e–
Negative electrode reaction:
C + y Li+ + ye– ⇔ LiyC
Total cell reaction:
LiMO2 + x/y C ⇔ x/y LiyC + Li1-xMO2
M = metals like Co, Mn, Ni, Ti, etc.
Normally x is about 0.5 and y is about 0.16, therefore x/y is about 3. [Jeff Dahn and Grant M. Ehrlich. “Lithium ion batteries”, Linden’s Handbook of Batteries, 4th edition, Thomas B. Reddy (Ed.), McGraw
Electrolyte and Solid-Electrolyte Interphase (SEI)
As mentioned earlier, non-aqueous electrolytes or organic electrolytes are used in lithium cells. Li-cells operate at comparatively higher voltages, up to 4.2 V per cell. Although bulky lithium salts such as lithium hexafluorophosphate (LiPF6), lithium hexafluoro arsenate (LiAsF6), lithium tetrafluoroborate (LiBF4), lithium perchlorate (LiClO4), lithium trifluoromethanesulphonate (LiCF3SO3), Lithium difluoro(oxalate)borate (LIODFB) etc. , are the actual electrolytes (sustaining electrolyte salts), they require suitable solvents stable at such higher voltage. Most of such solvents have high dielectric constants, facilitating easier ionic dissociation and the existence of highly concentrated Li-ions. Such solvents also serve as solvation sheaths for the stable existence of Li ions, thus decreasing the influence of the counter anions.
The disadvantage of having high dielectric constants is that they have higher viscosity values which result in impaired mobility of the ions. To overcome the lower ionic conductivity, low-viscous solvents are usually mixed with high viscous solvents. But, since the low-viscous solvents have lower ionic dissociation it becomes imperative to strike an optimum mixing ratio so that the mixture has both good ionic conductivity and good mobility. As non-aqueous solvents, mixtures of ethylene carbonate (EC) with less viscous linear alkyl carbonates such as dimethyl carbonate (DMC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) are used in commercially available LIBs.
The aprotic solvents are ethers, esters and alkyl carbonates: They are diethyl ether (DEE), tetrahydrofuran (THF), dioxolane, ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), diethyl carbonate (DEC), ethyl methyl carbonate (EMC), methyl formate, γ-butyrolactone (BL), methyl acetate, acetonitrile (AN), dimethyl sulfoxide (DMSO), dimethylformamide (DMF), methyl chloride, nitromethane etc.)
Liquid electrolytes are solutions of a lithium salt in one or more organic solvents, typically carbonates
Propylene carbonate (PC) cannot be used as an electrolyte if graphite is to be used as anode, since the former decomposes on graphite surface; PC used alone, without EC or small additions of LiBOB) Li bisoxalato borate), can cause degradation in graphite electrodes as it co-intercalates with lithium, resulting in exfoliation.
The electrolyte is invariant (same number of ions enter as leave the electrolyte during charge and
discharge). The electrolyte salt is usually dissolved in organic carbonate solvents. Each manufacturer has a different combination of solvents with ethylene carbonate (EC) being a common denominator for most
Solid-electrolyte interphase (SEI) layer formation is another important function performed by the electrolytes. When an alkali metal is immersed in a battery electrolyte, or when a negative potential is applied to a carbon or to an inert electrode immersed in the electrolyte, an SEI begins to form.
The SEI layer formed instantaneously upon contact of the metal with the solution, consists of insoluble and partially soluble reduction products of electrolyte components. SEI is the key factor which determines the safety, power capability, morphology of lithium deposits, shelf life, and cycle life of the battery. Good adhesion to the anode is important as well.
As emphasized above, practical primary or secondary alkaline or alkaline-earth batteries can be constructed only if the dissolution or corrosion of the anode can be stopped. Therefore, the electrolyte must be designed to contain at least one SEI precursor that reacts rapidly with lithium (or with the alkali-metal anode) to form an insoluble solid-electrolyte interphase. The products of the reduction of salt anions are typically inorganic compounds like LiF, LiCl and Li2O, which precipitate on the electrode surface. The reduction of the solvent is followed by the formation of both insoluble SEI components like Li2CO3 and partially soluble semi carbonates and polymers.
In the case of the carbon electrode, the voltage at which the SEI is formed depends on the type of carbon, the catalytic properties of its surface (ash content, type of crystallographic plane, basal-to-edge plane ratio), the temperature, concentration and types of solvents, salts and impurities, and on the current density. On the first charge of a lithium-ion battery, there is a loss of capacity called the “irreversible capacity loss” (QIR) mainly needed for the formation of the SEI.
In addition to the formation of the SEI, QIR may be caused by capacity loss associated with the formation of soluble reduction products (QSP).
Contamination-free SEI is essential for the long cycle life of the battery. It becomes even more important during cycling at high rates and at a greater depth of discharge.
The SEI in lithium hexafluorophosphate (LiPF6) and lithium hexafluoroarsenate (LiAsF6) solutions have a higher resistivity as compared to solutions of other salts. This is due to resistivity changes that contribute to species-controlled resistance which lead to the high interfacial impedance of the lithium anode in LiPF6 and LiAsF6 electrolytes. In addition, Li2CO3 is stated to be one of the best passivating agents for the enhancement of lithium-cycling efficiency [J Electrochem Soc.,164 (7) A1703-A1719 (2017)].
Separators for lithium-ion batteries
Separators for lithium-ion batteries are polyolefin microporous films and are generally uniaxially drawn polyethylene (PE) and polypropylene (PP), biaxially drawn PE or multiaxially drawn PP/PE/PP.
Raw materials for the active materials in lithium ion battery
Lithium ion batteries use different cathode materials. The anode is invariably carbon-based one, except a few like titanium-niobium oxide anodes, Li-Si alloy etc. The following Table and figure give some ideas about the different chemistries employed in these batteries.

Figure 12. A summary of some present and future electrode chemistry options for Lithium ion batteries. The proposed capacity of the Li(Si) is 50% of the theoretical capacity of the material, similar to the case found for some of the positive electrode material
[Credit: Yu Miao, Patrick Hynan, Annette von Jouanne, and Alexandre Yokochi, Energies 2019, 12, 1074; doi:10.3390/en12061074]
Table 1.
Characteristics of Lithium ion cells with different cathode materials
| Cathode Material | Li-Ni-Co-Al (NCA) | Li-Ni-Mn-Co (NMC) | Li-MnO2 (LMO) | Li-Iron Phosphate (LFP) | Li Titanate (LTO) | Li Cobalt Oxide (LCO) |
|---|---|---|---|---|---|---|
| Nominal Voltage of a cell (V) | 3.6 | 3.65 (2.7-4.2) | 3.8 | 3.25 (2-3.6) | 3.2 | 3.6 |
| Theoretical specific energy (Wh/kg) | 279 | 256 | 148 | 128 (373) | 293 (175) | 274 (370) (x=0.5) |
| Specific capacity for cathodes (Ah/Kg) Potential vs Li/Li+ (V) | 180-200 (3.8) | 200 | 148 (4.1) | 150-170 (3.45) | 175 | 274 (3.9) (x=0.5) |
| Specific Energy for cathodes (Wh/Kg) | 680-760 | 610-680 | 410-492 548 | 518-587 544 | -- | 546 |
| Safety | safe | Moderate | Safe | High | Very Good | Moderate |
Cathode materials in lithium ion battery
The cathode materials must satisfy several requirements on which the selection of the positive electrode material depends.
- To provide high capacity, these materials must incorporate a large amount of lithium as made.
- Further, the materials must reversibly intercalate with little structural change to permit long cycle life, high ampere hour efficiency, and high energy efficiency.
- To achieve high cell voltage and high energy density, the lithium exchange reaction must occur at a high potential relative to lithium.
- To facilitate high rate charge and discharge processes, the electronic conductivity and lithium ion mobility in the material must be high.
- The positive electrode material must not dissolve in the electrolyte and must be available at affordable cost. To minimize cost, preparation from inexpensive materials in a low-cost process is preferred
LiFePO4 is an exception to this rule. In LiFePO4, adequate lithium ion transport is achieved by use of electrode particles having a nanometre particle size. [Jeff Dahn and Grant M. Ehrlich. “Lithium ion batteries”, Linden’s Handbook of Batteries, 4th edition, Thomas B. Reddy (Ed.), McGraw Hill, pp. 26.6, 2011]
The positive active materials (PAM) in Lithium ion cells vary depending on the manufacturer. Cathode materials can be classified into three broad categories [Arumugam Manthiram, Nature Communications (2020) 11:1550]. They are:
Layered oxides - cathode materials in lithium ion battery
Several oxides of the general type LiMO2 (where M =vanadium, chromium, cobalt and nickel) crystallize in a layered structure in which the Li+ and M3+ ion occupy the alternate [lanes of the rock salt structure to give a layer sequence of O-Li-O-M-O.
In the layered oxide cathode LiCoO2, the large charge and size differences between Li+ and trivalent Co3+ ions lead to good cation ordering, which is critical to support fast two-dimensional lithium-ion diffusion and conductivity in the lithium plane.
Cathode materials require extremely high purity levels and must be almost entirely free of unwanted metal impurities – notably iron, vanadium and sulphur.

Figure 13. Simplified schematic of a layered structure in which there is alternate occupation of the
cation layers between the close-packed oxide ion layers.
[Credit: Robert A. Huggins, Advanced Batteries, Materials Science Aspects, Springer, New York, 2009, p.168]
The good structural stability along with high electrical and lithium-ion conductivity offers fast charge-discharge characteristics with good reversibility. With these features, LiCoO2 remains as one of the best cathodes to date with a high operating voltage of ~4 V. The LiCoO2 cathode solved
two major challenges associated with the sulfide cathodes pursued in the 1970s. It enabled not only a substantial increase in the operating voltage from <2.5 V to ~4 V but also the assembly of a cell without the need to employ a metallic lithium anode.
Spinel oxides - cathode materials in lithium ion battery
The second class of cathode is the spinel LiMn2O4. (General formula is AB2O4). Although this structure is generally pictured in cubic coordinates, it also has parallel layers of oxide ions on (111) planes, and there are both octahedrally coordinated sites and tetrahedrally coordinated sites between the oxide ion planes. The number of octahedral sites is equal to the number of oxide ions, but there are twice as many tetrahedral sites. The three-dimensional structural stability and high electrical and lithium-ion conductivity offer even faster charge-discharge characteristics for Li1–xMn2O4 with good reversibility compared with LiCoO2.
An important advantage on going from LiCoO2 to LiMn2O4 is the significant reduction in cost as manganese is two orders of magnitude lower in cost than Co. However, one critical issue with LiMn2O4 is the dissolution of manganese from the lattice into the electrolyte in presence of trace amounts (ppm levels) of H+ ions (acidity) in the electrolyte due to the well-known disproportionations of Mn3+ to Mn4+ and Mn2+ in acid.

Figure 14 . Schematic of the spinel structure in which the cations are distributed between the close-packed (111) planes of oxide ions among tetrahedral and octahedral sites [Credit: Robert A. Huggins, Advanced Batteries, Materials Science Aspects, Springer, New York, 2009, p.17].
The high voltage Lithium-Nickel-Manganese Oxide (LNMO) cathode material appears promising in next-generation batteries. But the stumbling block is the lack of an electrolyte that can handle the stresses of an LNMO-based battery. LNMO cathode based battery cells deliver results on par with other high-performance lithium based batteries, but at a considerably lower cost.
However, electrolyte manufacturers are getting very promising results from ongoing research & development that will, at some point, result in electrolytes that will function well in a LNMO battery cell. https ://blog.topsoe.com/the-cathode-material-for-next-generation-lithium-ion-batteries-is-ready
More recently, increasing the Ni content and lowering or eliminating the cobalt content in NMC cathodes is becoming much more prominent [Li, W., Erickson., E. & Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries, Nat. Energy 5, 26–24 (2020)].
Poly-anion oxides - cathode materials in lithium ion battery
The third class of oxides is polyanion oxides. Polyanion oxides like Fe2(MoO4)3 and Fe2(WO4)3 were found to undergo reversible insertion/extraction of two lithium ions per formula unit to give Li2Fe2(MoO4)3 or Li2Fe2(WO4)3 both by chemical and electrochemical methods
[Manthiram, A., Goodenough, J. B. Lithium insertion into Fe2(MO4)3 frameworks: comparison of M = W with M = Mo. J. Solid State Chem. 71, 349–360 (1987)].
Based on the works of Manthiram and Goodenough,
[Manthiram, A. & Goodenough, J. B. Lithium insertion into Fe2(MO4)3 frameworks: comparison of M = W with M = Mo. J. Solid State Chem. 71, 349–360 (1987). Manthiram, A. & Goodenough, J. B. Lithium insertion into Fe2(SO4)3 framework. J. Power Sources 26, 403–406 (1989).]
Exploration of lithium-containing phosphates as cathodes led to the identification of olivine LiFePO4 as a cathode [Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho-Olivines as positive electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997] in 1997.
But, the polyanion oxide class suffers from poor electronic conductivity. [Arumugam Manthiram, Nature Communications (2020) 11:1550].
Exploration of lithium-containing phosphates as cathodes led to the identification of olivine LiFePO4 as a cathode [Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho-Olivines as positive electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997] in 1997.
But, the polyanion oxide class suffers from poor electronic conductivity. [Arumugam Manthiram, Nature Communications (2020) 11:1550].
Manufacture of Cathode materials - Lithium ion battery
Earlier, the lithium metal oxide cathode compounds were made from lithium carbonate and a salt of the chosen metal by means of a series of chemical replacement reactions performed in solution. The desired product is precipitated and spray-dried.
LiCoO2 was first prepared by the conventional synthesis method indicated in the figure. Tricobalt tetraoxide (Co304) and lithium carbonate (Li2CO3) were mixed well, followed by calcination in the air flow at a temperature of around 950ºC. By this method, however, it was very difficult to prepare coarse particles of LiCoO2 and only fine particles with diameters of 1-3 pm could be obtained.
Fine active electrode materials are not desirable from the safety point of view. In the case of abuses such as external short circuit or crushing, fine particles with large specific surface area easily react at one time and all the cell energy is abruptly released within a very short time with accompanying temperature rise. In the worst case, the cell can catch fire [Yoshio Nishi, in Lithium ion Batteries, M. Wakihara and 0. Yamamoto (Eds.). page 192-193].
How lithium ion battery is manufactured? flowchart
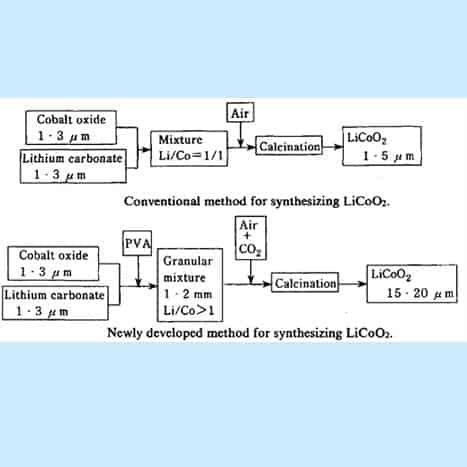
Figure 15. Flowchart for making Li-CoO2
[Credit: Yoshio Nishi, in Lithium ion Batteries, M. Wakihara and 0. Yamamoto (Eds.). page 192-193].
An improved process for synthesizing lithium cobaltite of larger particle size: The first point is that a small amount of PVA resin is added in the mixtures of raw materials (Co304 and Li2CO3)) to form granular pellets with a granulator. By sintering the pellets in an airflow containing suitable amount of C02 gas, lithium cobaltite particles with an average diameter of 20pm are synthesized. The second point is that we use a slightly excessive amount of lithium carbonate (Li2CO3) in the raw materials, so the Li/Co atomic ratio in the raw materials is greater than one. This procedure is also favourable for obtaining coarse particles, and in addition, the resulting LiCoO2 contains a small amount of residual Li2CO3.
The first point is that a small amount of PVA resin is added in the mixtures of raw ‘materials (Co304 and Li2CO3) to form granular pellets with a granulator. By sintering the Lithium cobalt oxide can be readily prepared by the high-temperature firing of a stoichiometric mixture of lithium carbonate Li2CO3 and cobalt oxide, Co3O4 or metallic cobalt at 600–800°C, then annealing the product at 900°C for many hours, all under an oxygen atmosphere.
It can also be obtained by calcination of hydrated oxide with lithium hydroxide up to 750–900°C.
A third method uses lithium acetate, cobalt acetate and citric acid in equal molar amounts, in water solution. Heating at 80°C turns the mixture into a viscous transparent gel. The dried gel is then ground and heated gradually to 550°C. (https: //en.wikipedia.org/wiki/Lithium_cobalt_oxide).
Some typical examples are: Sol-gel method
In a sol-gel process, the aqueous solutions of the reactants and a chelating agent solution are mixed. Slow evaporation of the solvent generates a sol and a moderate heating of the sol so obtained produces a gel. The latter is calcined at suitable temperature to get the desired product.
Example 1.
Synthesis of LiCoO2 from different complexing agents: The salts used were cobalt nitrate hexa hydrate (Co(NO3)2.6H2O, and lithium nitrate, anhydrous LiNO3. The gel was produced using four different complexing agents: citric acid, anhydrous (C3H4OH(COOH)3, glycine, (H2NCH2COOH); starch (commercial corn starch and gelatine).
Five solutions containing LiNO3 and Co(NO3)2.6H2O in 20 ml of water, with a proportion of Li:Co = 1.1:1 are prepared. A specific complexing agent is added to each solution: (i) citric acid (4.611 g) diluted in 5 ml of water; (ii) glycine (1.501 g); (iii) starch (1.250 g); (iv) gelatine (3.500 g) and (v) blank test.
The first four solutions were heated at a temperature of 70 to 80°C in a glycerine bath until the formation of the gel. The amount of time of this process is different for each gelling agent: (i) citric acid (5 hours), (ii) glycine (3 hours), (iii) starch (1 hour), (iv) gelatine (3 hours). The production of crystalline powders for all samples was performed in two stages in a muffle furnace: first with the firing the materials at 300°C for 20-30 minutes and later heating at 700°C for 24 h. [Bruno G. A. Freitas and others, J. Braz. Chem. Soc. 28, 11, Nov. 2017].
Example 2.
Prepared by a Sol-Gel Method
LiNO3 is first dissolved in citric acid solution. LiNO3, Ni(NO3)2.6H2O Co(Ac)2.4H2O and Mg(NO3)2.6H2O were used as starting materials of lithium, nickel, cobalt, and magnesium in LiNi0.7–𝑥M𝑥Co0.3O2 (0 ⩽ 𝑥 ⩽ 0.1), respectively. The amount of citric acid is equal to the total molar amount of Co, Ni, and Mg. Then, Co(Ac)2 4H2O, Ni(NO3)2 6H2O and Mg(NO3)2 6H2O were added to the mixture. The whole mixture was heated by water bath at 80∘C. During the heating process, a clear, pink solution without any precipitation formed. At last, the clear solution was slowly dried and turned into gel. The xerogel was dried, ground, and then heat-treated in an oven at 120°C for 12h.
The gel precursor was calcined at 500°C in air for 6 hours, and cooled to room temperature in a tube-furnace. The heat-treated products were ground in an agate mortar to obtain powders. And then the powder was calcined at 800°C for 12 hours. For fabrication of cathodes, the prepared products were first mixed with acetylene black and polyvinylidene fluoride (80: 8:12 in weight) in 𝑁-methyl pyrrolidone (NMP). The slurry obtained was then coated onto Al foil and dried at 80oC for 18 h for further roll pressing. . [Hailang Zhang, Advances in Materials Science and Engineering Vol 2014, Article ID 746341,]

Figure 16. Flow chart for sol-gel process to prepare lithium manganate
(Credit: Y.S. Lee, Y.K. Sun and K.S, Nahm, Solid State Ionics 109 (1998) 285 as given by, M. Pasquali, S. Passerini and G Pistoia, in Lithium Batteries, Science and Technology, ed. by G. A. Nazri and G. Pistoia, Springer, New York, (2009), p. 318)
Manufacture of Anode materials in Lithium ion battery
The encouraging path leading to LIBs with improved energy and power density is the selection of suitable anode materials which can provide high capacity and ease of diffusion of Li-ions into the anode, along with good cycle life and free from safety concerns.
Based on the precursor materials, carbon anodes can be classified into several types as given below.
The precursor material and the processing parameters determine the nature of carbon produced. Materials that can be graphitized by treatment at high temperature (2000 to 3000°C) are termed soft carbons.
Upon graphitization, the turbostratic disorder is removed progressively with increasing temperature, and strain in the material is relieved [T. Zheng, J. N. Reimers, and J. R. Dahn, Phys. Rev. B 51, 734 (1995)] Hard carbons, such as those prepared from phenolic resin, cannot be readily graphitized, even when treated at 3000°C. Coke-type materials are prepared at about 1000°C, typically from an aromatic petroleum precursor [Jeff Dahn and Grant M. Ehrlich. “Lithium ion batteries”, Linden’s Handbook of Batteries, 4th edition, Thomas B. Reddy (Ed.), McGraw Hill, pp. 26., 2011]

Figure 17. Carbon anode materials precursor classification
[Credit: Jeff Dahn and Grant M. Ehrlich. “Lithium ion batteries”, Linden’s Handbook of Batteries, 4th edition, Thomas B. Reddy (Ed.), McGraw Hill, pp. 26., 2011]
Goriparti divides anode materials of LIB into three categories depending on their reaction mechanism with lithium [Subrahmanyam Goriparti, Ermanno Miele, Francesco De Angelis, Enzo Di Fabrizio, Remo Proietti Zaccaria, Claudio Capiglia, J Power Sources 257 (2014) 421-443]
The intercalation/de-intercalation group
This category of anode includes carbonaceous and titanium oxides materials. The storage capacity that occurs through an intercalation path is closely associated with the surface area, morphology, crystallinity and its orientation. Soft carbons are commonly well accepted and used in the battery industry. It was seen that soft carbon is quite a mature technology, while hard carbon may present an interesting alternative solution especially for applications requiring high capacity such as in the electric vehicle sector. Titanium Oxide anodes are already used by some battery industries.


Graphene was also extensively reviewed. In particular, it was seen that their electrical properties make this material especially suitable for hybrid graphene/metal anodes (for example graphene with SnO2 and Fe2O3). Carbon nano-tubes (CNTs) were significant for their very interesting academic results, although production cost might hinder their application as anode active material in the battery industry for the future.


However, for large EV batteries, low-cost graphites are normally preferred due to cost considerations.
In a second category, alloying materials such as Si, Ge, SiO, SnO2 were described. These materials can provide larger capacities and high energy density compared to the previous group, by reacting with lithium in an alloy/de-alloy electrochemical mechanism. However, this process implies large volume expansion which results in a substantial capacity loss upon cycling. The reduction from bulk dimensions to the nanoscale, along with the realization of complex structures by the combination with conductive matrices, has been proposed to overcome the above-specified issues and to improve the overall anode performance.
Silicon and SnO2 and their composite with carbon are the most promising materials for applications in future lithium batteries, however, an inexpensive way for their mass production as anode materials is still necessary. On the other hand, Ge, although interesting for its electrochemical properties and excellent experimental laboratory results suffers of the drawback of being the fiftieth ranks element in terms of abundance in the Earth’s crust. Therefore, it seems not to be a good option for the lithium battery technology mass application.
In the third group, materials reacting with lithium in a conversion reaction fashion were described. In particular, metal oxides/ phosphides/nitrides/sulfides were considered. However, these materials are still far away from the large commercial lithium battery market, due to poor capacity retention and large potential hysteresis. Therefore, a variety of nano-structured forms of these materials have also been investigated to address the above-identified problems.
A nanotechnology is definitely a formidable approach for engineering the next generation of anode materials for lithium batteries. In order to utilize the described materials as effective anodes in commercial LIBs, especially for EV applications, more research work is however required. In fact, it is necessary to achieve both higher energy and higher power density together with the developing of inexpensive fabrication processes for large scale synthesis of nanosized materials. Furthermore, the investigation of the mechanisms ruling the interaction between lithium and the nanosized forms of the described materials along with the electron transport properties at the electrode/electrolyte interface is of crucial importance for the designing of the next generation of anode active materials engineered by nanotechnology.
Negative electrodes currently employed in lithium cells involve a solid solution of lithium in one of the forms of carbon. Lithium cells that operate at temperatures above the melting point of lithium must necessarily use alloys instead of elemental lithium. These are generally binary or ternary metallic phases. There is also increasing current interest in the possibility of the use of metallic alloys instead of carbons at ambient temperatures, with the goal of reducing the electrode volume, as well as achieving significantly increased capacity. [Robert A. Huggins, Advanced Batteries, Materials Science Aspects, Springer, New York, 2009, p.123].
Graphite is amphoteric, and either cations or anions can be inserted into it between the graphene layers. When cations are inserted, the host graphite structure takes on a negative charge. Cation examples are Li+, K+, Rb+, and Cs+. When anions are inserted, the host graphite structure takes on a positive charge, and anion examples are Br−,SO2– , SbF6–
The insertion of alkali metals into carbon was first demonstrated in 1926 [K. Fredenhagen and G. Cadenbach, Z. Anorg. Allg. Chem. 158, 249 (1926)] and the chemical synthesis of lithium–carbons was demonstrated in 1955 [D. Guerard, A. Herold, Carbon 13, 337 (1975)]. X-ray photoemission spectroscopy experiments showed that the inserted lithium gives up its electron to the carbon, and thus the structure can be viewed as Li+ ions contained between the carbon layers of the graphite structure
[G.K. Wertheim, P.M.Th.M. Van Attekum and S. Basu, Solid State Commun. 33, 1127 (1980)]. A general review of the early work on the insertion of species into graphite can be found in
[L.B. Ebert, Intercalation Compounds of Graphite, in Annual Review of Materials Science,
Vol. 6, ed. by R.A. Huggins, Annual Reviews, Palo Alto, CA (1976), p. 181].
The important factor in the purity of the anode material is the need to eliminate any oxygen-containing species on the surface because these would react with the electrolyte. To prevent this reaction, manufacturers bake the graphite at 1100ºC) in reducing or inert atmospheres. This increases the cost for other uses, compared to graphite. Carbon (90%) is mixed with several other ingredients to make the anode paste or slurry. As with the cathode, polyvinylidene fluoride (PVDF) is used as a binder (-5%), and a small amount of carbon black is added to ensure conductivity. In addition, n-methyl pyrrolidone (NMP) is used to solubilise the materials to form a uniform mixture. Pressure assures uniform grain size (Sandi 1999).
Lithium titanate (LTO) is gaining a lot of interest. LTO cells operate at a lower temperature than other chemistries and offer high power density. However, such cells suffer from having a lower nominal voltage, in the range of about 2.2–2.3 V per cell. [Norio Takami, Hiroki Inagaki, Yoshinao Tatebayashi, Hidesato Saruwatari, Keizoh Honda, Shun Egusa, J Power Sources 244 (2013) 469-475]
The electrode material, typically graphite, expands by 10 % during the charging process. The graphite regains its original volume when the lithium ions deintercalate. The lithium ions would not only be intercalated into the graphite if aluminium is used but also inserted into the conductor, thus forming an aluminium-lithium alloy. The reverse process would occur during discharging. The aluminium would be degraded after a few cycles and would be useless as a current collector.
However, if the negative electrode is made from lithium titanate instead of graphite, the situation dramatically changes. The electrode potential of Li4Ti5O12 is about 1.4 V higher than that of graphite (cell voltage is around 1.4 V lower, 2.2 V as against 3.6 V). This would prevent the lithium ions from being intercalated into the aluminium. Therefore, aluminium is preferred over copper for cost-related and weight-related reasons. Li4Ti5O12 is employed mainly in stationary applications because of its lower cell voltage. [Călin Wurm et al., in Lithium-Ion Batteries, Reiner Korthauer (ed), Translated by Michael Wuest et.al., Springer, 2018. pp. 57].
Process for producing lithium titanate: A mixture of titanium dioxide and a lithium compound (any one of these: lithium carbonate, lithium hydroxide, lithium nitrate, and lithium oxide) is pre-sintered at a temperature of between 670°C and 800°C. A compound consisting of TiO2, and Li2TiO3 or a compound consisting of TiO2, Li2TiO3, and Li4Ti5O12 is obtained. The compound is then sintered at a temperature in the range of 800 to 950°C. [Tetsuya Yamawaki et.al., U S Patent 6,645,673 B2, 2003 Assigned to Toho Titanium Co., Ltd., Chigasaki]
Toshiba’s SCiB™ Rechargeable Battery (https://www.scib.jp/en/)
SCiB™ uses lithium titanium oxide (LTO) in its anode to achieve safety, long life, low-temperature performance, rapid charging, high input/output power and large effective capacity. SCiB™ has found wide applications in vehicle, industrial and infrastructure applications, including automobiles, buses, railroad cars, elevators and power plants.
Lithium ion battery separator production
Two types of process are available: wet and dry. The Japanese manufacturers use a wet process in which the polymer is dissolved in oil. The oil is then evaporated to leave a porous film. They use polymers of ultrahigh molecular weight to produce Celgard, three layers of blown polymer film are laminated, drawn down, and annealed below the melting point to control the polymer structure. The sheet is then rapidly stretched to obtain porosity.
Two types of process are available: wet and dry. The Japanese manufacturers use a wet process in which the polymer is dissolved in oil. The oil is then evaporated to leave a porous film. They use polymers of ultrahigh molecular weight to produce Celgard, three layers of blown polymer film are laminated, drawn down, and annealed below the melting point to control the polymer structure. The sheet is then rapidly stretched to obtain porosity.
[Pekala, R.W., et al., 2000, “Separators: An Overlooked Opportunity to Enhance Battery Performance?,” 17th International Seminar and Exhibit on Primary and Secondary Batteries, Ft. Lauderdale, Fla., March 6-9]
This process is very sensitive to operating conditions and even varies with material batches, so careful control is necessary [Linda Gaines and Roy Cuenca, Cost of Lithium ion batteries for Vehicles, ANL Report ANL/ESD-42, May 2000, pp. 20 ].
However, the additional thickness required in separators for EV/HEV cells compensates for the reduced strength. [Y. Nishi, in: M. Wakihara, O. Yamamoto (Eds.), Lithium Ion Batteries, Wiley/VCH/Kodansha, Tokyo, 1998, p. 195.
P. Arora, Z. Zhang, Chem. Rev. 104 (2004) 4419].
In addition to conventional characteristics such as good mechanical strength, electrolyte permeability, these micro porous separators display a protective property during cell abuse. For example, if the cell temperature rises abnormally because of an excessive overcharge, for example, the heat generated softens PE and closes the micropores in the film. This is called separator “shutdown”. Once shutdown occurs, ionic transport between the electrodes is effectively stopped and current ceases to flow. If the separator can retain mechanical integrity above its shutdown temperature, it can provide a margin of safety to the device; otherwise, the electrodes can come into direct contact, react chemically, leading to thermal runaway.
However, it is possible that due to thermal inertia the temperature can continue to rise even after shutdown. Under such conditions the separator would melt and short the electrodes, leading to violent reactions and heat generation. This phenomenon is called “meltdown” or “breakdown” of the separator. Therefore, in order to ensure the safety of the cell, the difference between the “shutdown” and “meltdown” temperatures should be as large as possible.
Separators made entirely of high-density polyethylene melt at 135°C and lose mechanical integrity above this temperature. However, separators made by laminating layers of polypropylene and polyethylene maintain mechanical integrity at least up to 165°C, the melting point of polypropylene. It is interesting to note that although ultrahigh molecular weight polyethylene melts at 135°C, separators made from this material retain their mechanical integrity up to at least 180°C as the viscosity of the material is such that it maintains physical integrity.
Shutdown separators are reliable and lithium-ion battery manufacturers are increasingly opting for their incorporation in their products. The most common shutdown separators have high molecular weight polypropylene blended with super-high molecular weight polyethylene. Here, the unique shutdown property of polyethylene is combined favourably with the high mechanical integrity of polypropylene at elevated temperatures. Because the shutdown is irreversible, once actuated, these separators leave the cells permanently damaged. [P.G. Balakrishnan, R. Ramesh, T. Prem Kumar, J. Power Sources. 155 (2006) 401–414]
Other materials in Lithium ion battery
There are other materials like current collectors like aluminium, nickel and copper foils, binders like styrene-butadiene copolymer (SBR), and polyvinylidene fluoride (PVDF), electrolytes and solvents, cathode conductive additives, separator.
Advantages & Limitations of the Lithium-ion Battery - Lithium ion cell manufacture
Anode to cathode weight ratio
It is very important that no lithium metal be formed during the cell operation. The deposition of the metal forms dendrites that internally short the cell. The voltage control during charging and cell balance help to reduce this problem to a very great extent. The main method to control lithium deposition is the ratio of the anode to the cathode capacity of the individual plates in the cell. The anode electrode has about 10% higher usable capacity than the cathode. This prevents lithium metal deposition on the anode during charge, as the cathode determines the capacity of the cell. If lithium metal deposits on the electrode surface, it reacts with the electrolyte and could initiate thermal runaway.

Figure 21. Anode and cathode capacity ratio in Lithium ion cell
(Credit: Ralph J. Brodd and Kazuo Tagawa, in Advances in Lithium-Ion Batteries, Walter A. van Schalkwijk and Bruno Scrosati (Eds), Kluwer Academic Publishers, New York, pp. 272, 2002.)
Lithium ion cell assembly processes
The cell assembly processes for a Lithium ion battery require precision and accuracy when coating the positive and negative electrode stock coated with the active material. The coating process is a critical element in ensuring high capacity, high-reliability product. If the coatings are of poor quality only low-performance batteries will be produced. The initial steps in the preparation of the active mass determine the outcome.
Cohen and Gutoff [E. Cohen and E. Gutoff, Modern Coating and Drying Technology, Wiley-VCH,
New York, 1992] describe a methodology to arrive at the best coating technique for a particular application, based on the rheology of the coating slurry, required precision and speed of coating.

Figure 22. Anode and cathode coating process
(Credit: Ralph J. Brodd and Kazuo Tagawain in Advances in Lithium-Ion Batteries, Walter A. van Schalkwijk and Bruno Scrosati (Eds), Kluwer Academic Publishers, New York, pp. 273, 2002.)
Flow chart for manufacturing of Lithium ion battery

Figure 23. FLOW CHART FOR MANUFACTURE OF LITHIUM ION CELLS
[Ralph J. Brodd and Kazuo Tagawa in Advances in Lithium-Ion Batteries, Walter A. van Schalkwijk and Bruno Scrosati (Eds.), Kluwer Academic Publishers, New York, pp. 271, 2002.]

Credit: Electropaedia https: //www.mpoweruk.com/battery_manufacturing.htm
Figure 24. Flow chart for manufacture of electrodes from raw materials
Lithium ion cell assembly




The Lithium ion battery manufacturers aim at the following points while assembling cells:
- The designs for the Lithium ion cell must result in uniform current density throughout the electrode area.
- To ensure good contact between the active materials (AM) and the current collector
- Large surface area electrodes are employed to give the cells high rate performance. This reduces the polarization, i.e., voltage losses due to the kinetics of the electrode reactions and lowers the voltage drop across the separator.
The pore structure and the combination of conductive carbons give good inter-particle contact of the active material.
Good contact is essential between the active materials, the conductive carbon and the current collector, for full utilization of the active materials and for good efficiency during high rate performance.
The cobalt cathode mix is prepared from LiCoO2 (a black powder) + PVdF binder (a white semi-crystalline fluoropolymer thermoplastic) + N-methyl pyrrolidone (NMP, a colourless organic liquid) as a solvent. LICoO2 being nonconductive, a conductive diluent, invariably a carbon black, is added to enhance the conductivity of LiCoO2.
The ratio and amounts of the materials are determined by the cell design and the size of the mixer. An intensive mixing procedure is used to dry blend the non-conducting active material and the carbon before adding the coating solvent and binder.
The mixture is dry blended to give a uniform coating of the particles of the active material with a thin film of the conductive carbon so that the electrical contact between the AM and the current collector grid (aluminium foil, 20 mm in thickness) is improved, thus ensuring full utilization of all AM. The polymer NMP is dissolved in the coating solvent in a separate container. The dry mix blend and the solvent solution are then combined to form a slurry.
The solvent additions are used to adjust the viscosity of the slurry (or paint) for the coating operation. Polyvinylenedifluoride (PVdF) is the binder of choice and the solvent is N-methylpyrollidinone (NMP). The slurry from the mixing operation is placed in sealed containers, which serve as the reservoir and transfer medium for the coating operations. Precise amounts of coating slurry are pumped from the storage container with a gear pump, or similar precision pump, to avoid any entrainment of air in the fluid going to the coating head.
The anode mix is prepared in a similar manner with hard carbon, PVdF binder and NMP. This mix is coated on a copper foil used as the grid (10 mm in thickness).
Coating is done on both sides to about 100 mm in thickness for both anodes and cathodes. By decreasing the coating thickness an increase in total surface area is achieved for a definite volume of the cell. The organic electrolytes used possess lower conductivity compared with aqueous ones and so this higher surface area will facilitate for a high power discharge cell.
The electrode thickness depends on the maximum power required. A unique feature of the Lithium ion battery manufacturing technology is that it allows a wide range of power/energy ratio designs with the same electrode manufacturing technology [Broussely, Nazri pp 651]. But the suitable current collection and tabbing, cell shape and design are important.
Cell Assembly: The coated foil passes through an oven to evaporate the solvent and leave a precise amount of active mass on the foil. Many coating solvents are classed as hazardous and cannot be released to the atmosphere. As a cost-saving measure, the solvent is generally recovered for reuse in the process. To avoid any contamination of the environment the solvent may be incinerated.
Most Lithium ion cells are cylindrical in shape. The jelly roll is flattened to obtain elements for prismatic cells.
The prismatic cells are favourable for better volume filling, but are liable for bulging on cycling or ageing. The cylindrical cell cans offer better mechanical strength, good dimensional stability and uniform pressure in the elements.
The coating operation produces interrupted coating to match the length of the coil. The winding machines are designed to operate automatically for use with dried jumbo rolls of cathodes and anodes and separator (25mm or less in thickness, either PP or PE or mixed).
The operations start by welding the tabs onto the uncoated section of the foils. The winding machine then cuts the strip to the proper length and winds the combination anode-separator-cathode into a tight coil or bobbin in jellyroll fashion. As the wound core increases in diameter, the winding machine automatically compensates to maintain constant tension as the coil increases in diameter for close tolerance on the diameter. The elliptical wind for prismatic cells is a more complex and slower process.
After winding, the coil is checked for internal shorts before being inserted into the can. The steel cans should be clean and nickel-plated to provide a stable surface and minimize can corrosion before cell assembly. The anode lead is welded to the bottom of the can and the cathode lead is welded to the safety vent. The electrolyte is added to the half-assembled cell. Assembly is finished with the crimping of the top cover.
Early rejection of potential cell faults is an economic measure and prevents more work on bad cells. The bobbin is inserted into the can so that the can provides constant pressure to hold components of the element close together, thus eliminating any chance for voids between them. Some manufacturers may insert a mandrel to stabilize the centre of the coil.
Unless all operations are carried out in a dry room or dry box, the absorbed water in the active materials must be removed by heat and vacuum before the electrolyte filling process.
Precision vacuum filling of electrolyte is done to ensure that the electrolyte permeates and completely fills the available porosity in the separator and electrode structures. Precision pumps meter the calculated volume of electrolyte needed for good cell operation. Invariably all manufacturers employ LiPF6 (an inorganic white crystalline compound) as the electrolyte and cyclic (EC, ethylene carbonate) or linear carbonates (DMC, dimethyl carbonate, DEC, diethyl carbonate, or EMC, ethyl-methyl carbonate, etc.) are the solvents for this electrolyte salt.
Electrolytes based on solvent mixtures of ethylene carbonate (EC) with dimethyl carbonate (DMC) and/or diethyl carbonate (DEC) are commonly used for lithium ion batteries in combination with “4 V” cathodes (cobaltate, nickelate and manganate) because of the high oxidation potential of the solvents.
After filling the cell with electrolyte, the cell is sealed by controlled compression of a polymer gasket or grommet placed between the cell can and the top plate. The pressure on the polymer gasket seal is controlled to keep it within the elastic limit of the polymer. If the elastic limits are exceeded, the polymer cold flows and compromises the seal.
Each manufacturer uses a somewhat different mechanical construction to seal the cells but the final results are essentially identical. Typically, a shoulder or ledge is formed near the top of the cell. This serves as the base for the seal and to hold the jellyroll in place and prevent telescoping or changing position of the wound bobbin under the influence of vibration and shock.
Any shift in position causes a change in the current distribution and results in poor cycle life or lithium plating in high-performance cells. The cell top plate seal contains a vent, a positive temperature coefficient element (PTC) and a current interrupt (CID) safety devices. Both the CID and PTC are safety devices designed to activate and prevent dangerous temperatures and pressures from developing internal to the cell. Each lot of devices is checked for proper operation before incorporation into the top assembly.
After the seal is applied the cells can be washed, jacketed and labelled. They are given a serial number to trace the day of manufacture and to identify all cell components (electrode materials, electrolyte, separator and the like). The information on capacity and voltage is stored with the cell number and used later to match cells for pack assembly.
The cells can be laser welded with glass to metal seals to provide long-lasting hermetic seals. With the larger cells, greater care must be exercised to ensure safe operation, even under abuse conditions.
While the processes, above, are depicted for small sealed cells used in portable electronics, the process for larger industrial batteries for energy storage, space and EV applications follow the same general outline.
Lithium ion battery - Formation and ageing
As assembled, Li ions are not doped into the anode carbon and so the cell shows no voltage. During initial charging, a part of Li ions from the PAM LiCoO2 is undoped to become Li1-xCoO2 and these Lithium ions are doped into the carbon anode (Cy) to become LixCy. When the charging voltage reaches 4.1 to 4.2 V, the value of x is about 0.5. (i.e., 50 %) indicating that 50 % of Li from LiCoO2 has been used.
Another aspect to be noted is that a part of the doped Lithium ions does not return and remain in the anode. where, x-dx Lithium ions stay without contributing to the capacity. This is about 10 to 20 % irreversible lithium, which means that the efficiency of the initial charge is 80 to 90 %. From the second cycle, the irreversible amount does not increase and the cell shows the 100 % capacity designed by the manufacturer.
After washing and jacketing, but before the start of the formation process, the voltage and impedance of all cells are recorded to sort out any defective cells. The cells are then charged for the first time
(initial charging or formation charging). The conditions of the first charge are important for at least two reasons:
1) the solid electrolyte interphase (SEI) layer forms on the anode to protect it from reacting spontaneously with the electrolyte during normal cell operation, and 2) it establishes the good electrical contact between the active materials and the electrolyte. The first charge follows the manufacturer’s recommended procedure for charging the cells but often starts at a lower current and then increases to the normal charging current at about one-third of the way into the charge period. Cells may continue cycling within the voltage limits for charge and discharge for one or two more cycles after formation.
After formation or cycling, cell voltage and capacity are measured and stored for later use in the cell selection process. The ageing period varies between two weeks and one month, depending on the manufacturer. The voltage of the cells is measured again after storage. Differences in voltage at the start and end of the storage period are used to sort-out cells with “soft-” or “micro-” shorts. Cells with internal shorts will have a lower voltage after storage and separate themselves from the normal voltage and capacity distribution. It may be necessary to evacuate the larger cells after formation to remove the formation gases.
For a detailed description of assembly process, readers are referred to
- Kaoru Nakajima and Yoshio Nishi Chapter 5 in: Energy Storage Systems for Electronics, Ed Tetsuya Osaka and Madhav Datta, Gordon and Reach Science Publishers, Amsterdam, 2000.
- Lithium-Ion Cell Production Processes, Ralph J. Brodd and Kazuo Tagawa, Chapter 9 in: Advances in Lithium-Ion Batteries, Walter A. van Schalkwijk and Bruno Scrosati (Eds), Kluwer Academic Publishers, New York, pp. 273, 2002.
- Lithium Batteries, Science and Technology, ed. by G. A. Nazri and G. Pistoia, Springer, New York, 2009.
- Lithium-Ion Batteries, Reiner Korthauer (ed) (2018), Translated by Michael Wuest et.al., Springer, 2018.
- Kazuo Tagawa and Ralph J. Brodd, Production Processes for Fabrication of Lithium-Ion Batteries in Lithium-Ion Batteries, Yoshio M., Brodd R.J., Kozawa A. (eds) Springer, New York, NY. https: //doi.org/10.1007/978-0-387-34445-4_8]
- Zhang Z., Ramadass P. (2012) Lithium-Ion Battery Systems and Technology. In: Meyers R.A. (ed) Encyclopaedia of Sustainability Science and Technology. Springer, New York. https: //doi.org/10.1007/978-1-4419-0851-3_663
- The Handbook of Lithium-Ion Battery Pack Design Chemistry, Components, Types and Terminology, John Warner, Elsevier, 2018